
Doctor of Osteopathic Medicine
Cancer screening in asymptomatic healthy populations is problematic. We have currently a menu of testing and guidelines for mammograms and colonoscopies, low dose lung CT scans, pap smears but really at the end of the day – none of these are perfect at finding early malignancies and of course some of these screenings are invasive and many have a high false positive rate leading to more testing and of course worry. In addition, for many cancers, there are no screening / early detection interventions that are used routinely.
What if there was a simple blood test that could test for 50+ types of cancer and identify if one was present and tell you the organ system in which the cancer was located?
Well this test does exist. How good is liquid biopsy at solving the problem of finding malignancy early enough to make a difference in morbidity and mortality from cancer? I dove into the state of literature for these tests and will outline for you the current utility of these tests.
The tests we are talking about use something called “methylation signatures” on DNA that is sitting in the blood and not inside of a cell- this is called cell free DNA. Some cancer cells die and release DNA into circulation as part of the growth cycle of malignancies. This DNA looks different from normal cell DNA. Our DNA is spun around proteins called histones and our cells will express the parts of the DNA that are unraveled involving methylation. Methylation is a process by which the DNA is tagged with a “methyl group”- One Carbon and three Hydrogens which give the cell instruction on what to do and how to act based on where they are in the body. Cancer cells have a specific pattern of methylation on the DNA which triggers them to behave abnormally- and go rogue. This pattern can be tested for in the DNA floating around in your blood. Researchers can now test your blood to see if there are methylation patterns of cancer and if so – what type of cell that DNA came from.
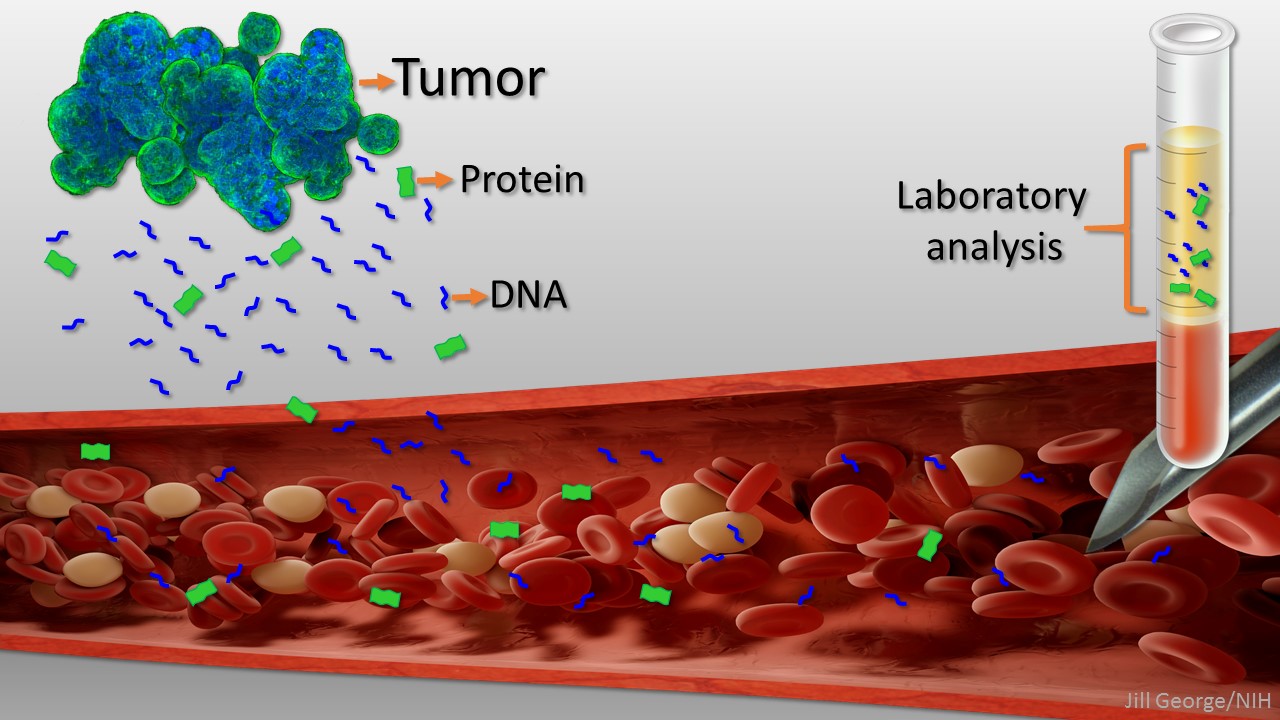
These tests can fairly definitively RULE OUT the presence of cancer DNA with over 99% certainty in a population where the incidence will be relatively low- asymptomatic healthy people. So if you have one of these tests and it is negative, we can pretty much say that you do not harbor a malignant tumor.
These tests do pose a conundrum if your test comes back positive. Depending on the type of cancer and stage of the cancer, there is a higher than ideal risk that it is a false positive. The more advanced stage of cancer, the less likely a false positive. For certain cancers that tend to not spill out as much cell free DNA, there is a higher false positive rate as well. Across all cancers and stages, on average the sensitivity is about 40% in healthy symptom free populations which means that more often a positive test will be a false positive rather than a true positive. If a positive test occurs, we will know what organ system, so additional testing at that point needs to be done to either rule in cancer (true positive) or rule out cancer (false positive). The other issue is that the liquid biopsy test does not necessarily pick up cancer earlier than other screening tools for the cancers- that we have a screening test for. In my mind therefore, this test is not a replacement for the standard screenings we have in place but rather could be used adjunctively at this time. This can add an extra layer of screening for the cancers we have recommendations for as well as screening for cancers we do not have any screening tests for.
The other issue that comes up with liquid biopsy is cost of testing. At this time the testing is not covered by health insurance and probably will not be until more population based studies are done showing value in reducing cancer morbidity and mortality. These tests are however available as an out of pocket expense or with health savings and could be done annually as an additional cancer screening tool.
When contemplating this type of test, I recommend going into it with the mindset of – if there is a positive, we will need to take further “screening” action to confirm or negate the results. If negative however, we can be confidently reassured that no malignancy is present.